The DNA Mismatch Repair ('MMR') pathway provides cells with a highly conserved DNA repair mechanism that plays a critical role during DNA replication, repair and recombination. MMR contributes to the maintenance of genome stability by correcting DNA base mismatches and insertion/deletion (indel) loops that occasionally arise during normal DNA replication. Base pair mismatches occur when incorrect nucleotides are inserted into the newly synthesized DNA strand and then escape the proofreading function of high-fidelity DNA polymerases. Indel loops more commonly arise in the context of microsatellites - highly polymorphic short repetitive DNA sequences. Alterations in the repeat length of such sequences, referred to as Microsatellite Instability ('MSI'), are used as markers of MMR deficiency ('MMRd') in human cancers.
DNA Mismatch Repair Inhibitors
Mechanistically, MMR can be divided into four steps:
-
Mismatch recognition by MSH proteins
-
Recruitment of MLH proteins that connect the mismatch recognition signal to where DNA strand scission begins
-
Excision of the errant DNA strand
-
Re-synthesis of the excision gap using the remaining DNA strand as a template
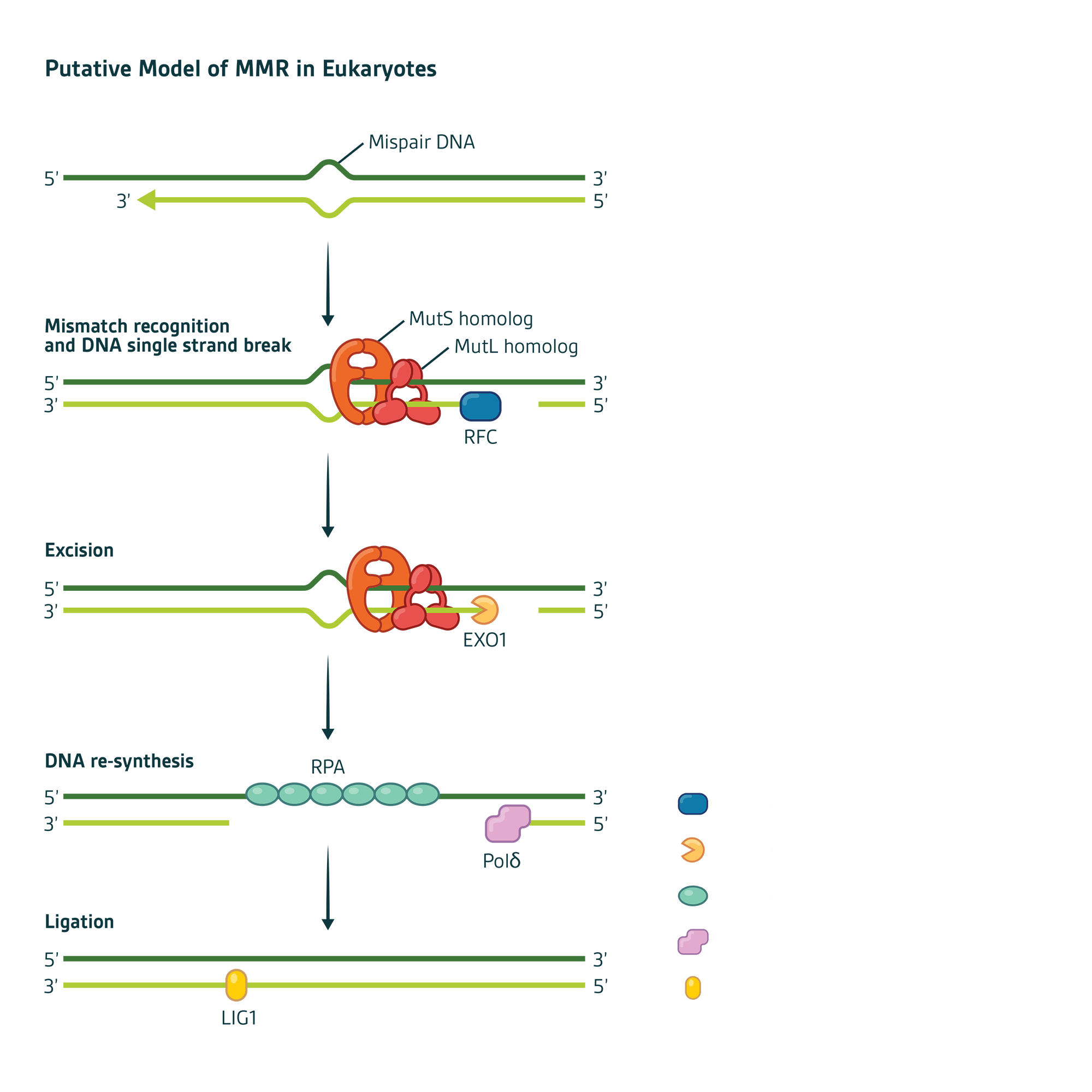

In humans, mismatch recognition by hMutSα ('MSH2-MSH6') or hMutSβ ('MSH2-MSH3') initiates the MMR pathway. Binding of hMutSα or hMutSβ to the mismatch site results in the recruitment of MutLα ('MLH1-PMS2') to form a ternary complex whose protein-protein and protein-DNA interactions are modulated by ATP/ADP co-factors.
DNA MMR is a tumour suppressor pathway that is lost in up to 40% of sporadic cancers. MMR-deficient cancers are commonly characterised by the accumulation of DNA mutations at higher rates than normal cells and other tumours. Cancers that harbour two or more of five routinely tested microsatellite markers are described as high frequency MSI ('MSI-H'), and MSI analysis is a clinically used diagnostic biomarker for MMRd tumours. MSI status has been associated with high Tumour Mutational Burden ('TMB') shown to correlate with the creation of new protein amino acid sequences ("neoantigens") that are substrates for antigen processing and presentation, stimulating immune activation. Tumours with a greater number of neoantigens are more immunogenic and prone to immune surveillance that correlates with higher survival rates in colorectal cancer patients.
Patients with MSI-H tumours have an increased likelihood of responding to immunotherapy, such as immune checkpoint blockade supporting a rationale for immunotherapy-based treatment strategies. (See Figure 1)
Figure 1
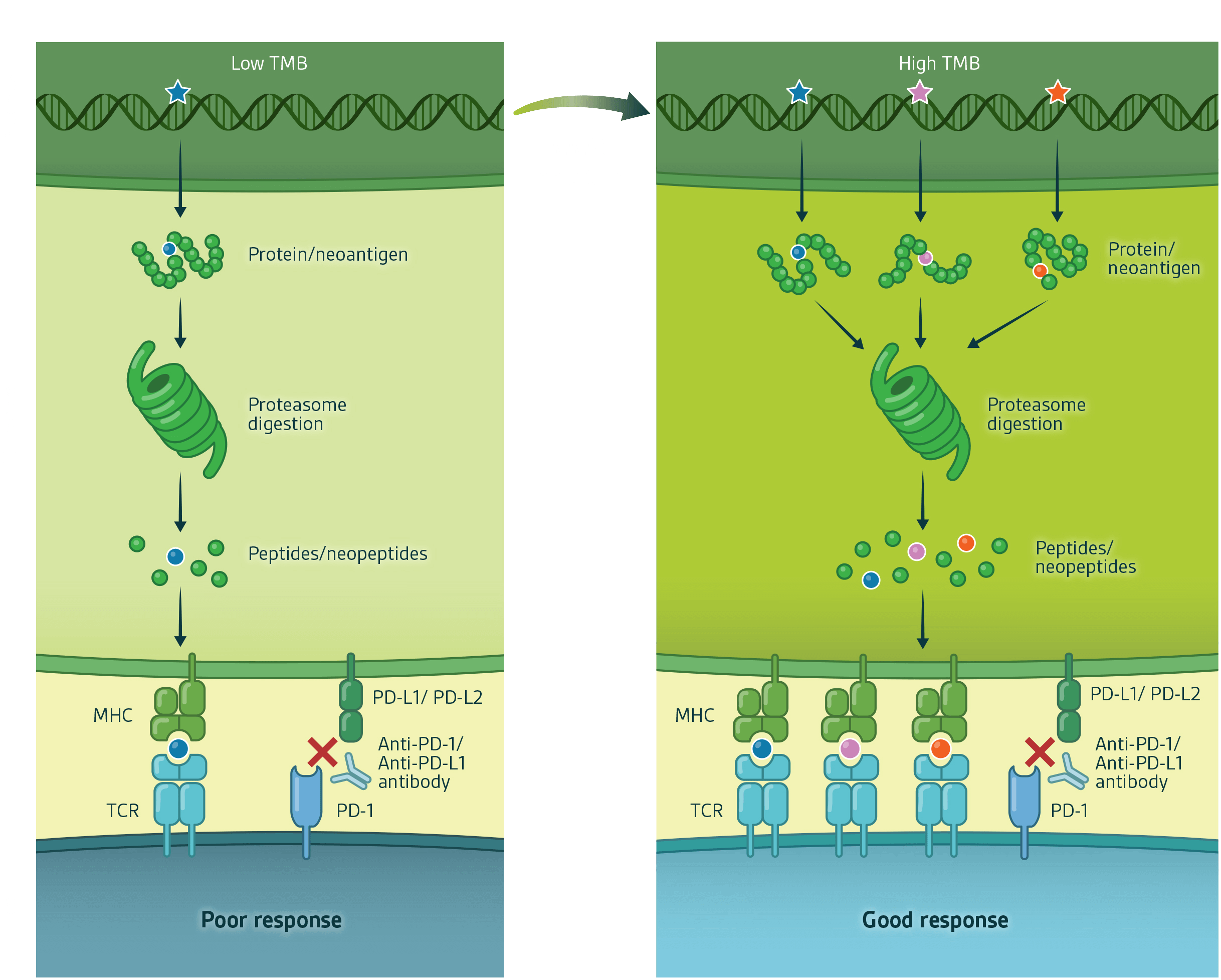
Tumour mutational burden/MSI status, neoantigen production and immunological response
Antigens derived from wild-type proteins are usually recognised as “self” and do not generate an immune response. When certain mutations occur in tumour DNA, such as nonsynonymous somatic mutations, a novel protein sequence forms to produce a neoantigen which can ultimately be processed and presented on the surface of the tumour cells in the context of MHC molecules. Altered peptide sequences derived from neoantigens and presented in the context of MHC may then be recognised by T cells leading to an anti-tumour immune response.
Tumours with high tumour mutational burden (particularly those originating from MMR deficiencies with an MSI-High status) are more immunogenic and have the potential to generate a larger number of MHC-presented neoantigens. Such tumours are therefore more likely to lead to an enhanced immune cell recognition compared with tumour cells with low tumour mutational burden.
Anti-tumour immune surveillance can still be restrained by immune checkpoints, such as PD-L1 that inhibit T cell activation. Thus, the combination of immune checkpoint blockade together with an increased neoantigen repertoire can elicit powerful immune responses leading to tumour rejection by the patients’ immune system.

Relationship between TMB, MSI and neoantigen generation
Immune checkpoint inhibitors offer a significant therapeutic advance in the treatment of MSI/MMRd cancers. More specifically, inhibitors of PD-1 have been approved by the Food and Drug Administration (FDA) for patients with MMRd or MSI-H metastatic colorectal cancer based upon the significant survival benefit they provide. Importantly, the FDA has approved the use of pembrolizumab (Keyruda®) in MMRd/MSI-H cancers regardless of histological tumour type.
It is commonly accepted that sustained clinical responses after treatment with CPIs require the existence of tumour neoantigens and infiltration of T cells that recognise neoantigens. Higher neoantigen load is also associated with response to immune checkpoint blockade in patients with melanoma and non-small-cell lung cancer. Increased and diverse neoantigen expression requires high TMB, and several large studies demonstrate that high TMB correlates with enhanced CPI responses and improved overall survival in tumours such as urothelial carcinoma, non-small-cell lung cancer and small-cell lung cancer.
There is an overwhelmingly strong rationale (both biological and clinical) to develop a therapeutic intervention against key components of the DNA MMR pathway. Treatment with MMR inhibitors in combination with CPI immunotherapies could clearly provide clinical benefit by reawakening the patients’ anti-tumour immune response by effectively turning immunologically ‘cold’ tumours ‘hot’.